The original version of this story appeared on ExplorersWeb.
The U.S. Food and Drug Administration (FDA) announced a new injection treatment this week for treating severe frostbite. Approved for adults, Aurlumyn (iloprost) can reduce the risk of amputation following frostbite, officials said.
“This approval provides patients with the first-ever treatment option for severe frostbite,” said Norman Stockbridge, M.D., Ph.D., director of the Division of Cardiology and Nephrology in the FDA’s Center for Drug Evaluation and Research.
“Having this new option provides physicians with a tool that will help prevent the life-changing amputation of one’s frostbitten fingers or toes.”
Severe frostbite is the deepest stage of tissue damage from prolonged exposure to extreme cold. It’s characterized by loss of touch and temperature sensation. The tissue turns black before blistering badly, and has long been a common injury among mountaineers and other winter sports athletes.
No Amputations Required
While tissue regeneration from severe frostbite was previously possible with “optimal” medical treatment, according to the U.S. National Institutes of Health (NIH), injury reversibility was limited.
But the FDA’s case studies on iloprost, which opens blood vessels to prevent clots, were promising. The agency placed 47 adults with severe frostbite into three groups.
“Group 1” received iloprost intravenously for 6 hours daily, for up to 8 days. The two other groups received other treatments unapproved for frostbite, given with iloprost (Group 2) or without iloprost (Group 3).
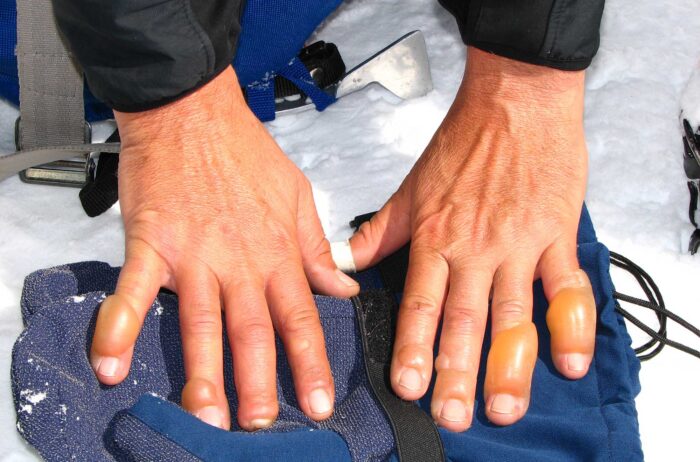
Bone scans followed to allow doctors to diagnose the potential need for amputation. Zero out of 16 patients in Group 1 needed amputation. Group 2, the patients with iloprost along with the unapproved medications, fared better than Group 3.
Follow-up appointments proved consistent with the initial bone scan results, the FDA said. The most common side effects of Aurlumyn include headache, flushing, heart palpitations, fast heart rate, nausea, vomiting, dizziness, and hypotension (low blood pressure), according to the FDA.
Actelion Pharmaceuticals US, Inc. earned the approval. The Johnson & Johnson subsidiary specializes in treating pulmonary arterial hypertension (PAH), according to its website, and markets several drugs approved for that use.








